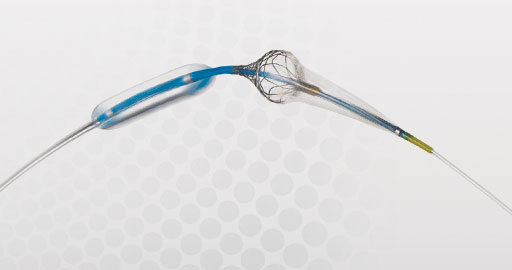
Paladin®
Carotid Post-Dilation Balloon System with Integrated Embolic Protection
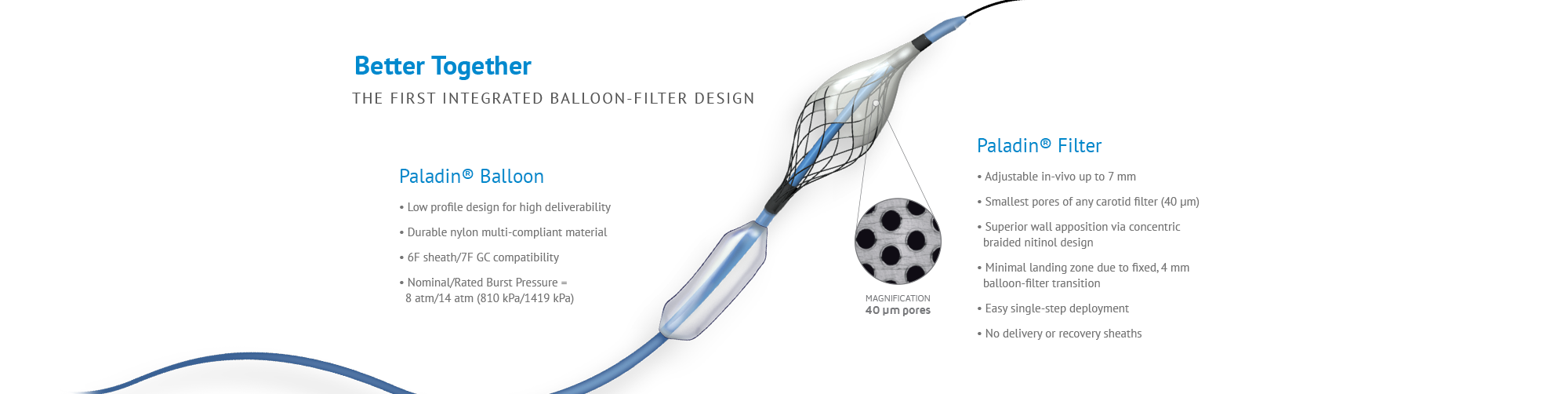
Despite the widespread use of embolic protection devices, the risk of stroke remains one of the biggest challenges in carotid artery stenting, especially in the critical procedural stage of post-dilation. The Paladin Carotid Post-Dilation Balloon System with Integrated Embolic Protection contains an angioplasty balloon and an integrated 40 μm filter, coupled together for the first time. Whether the operator chooses to use a distal filter or a proximal occlusion as a primary method of embolic protection, the additional protection provided by the Paladin filter can provide an added measure of protection and efficiency in carotid stent procedures.
For product inquires in Europe:
sales@contegomedical.com
The Paladin System is CE marked and available in select European markets. Physicians are advised to consult the local product IFU prior to use.
Easy Single-Step Deployment & Removal



Stroke Protection
When it Matters Most
Ischemic stroke risk remains unacceptably high in carotid stent procedures, even with the use of conventional embolic protection devices. Some groups such as symptomatic patients and octogenarians are at higher risk than others.¹
Size Matters
Paladin Captures What Others Miss2
- The Paladin filter captures smaller emboli. Paladin pore sizes are ≈ 40 μm while commercially available filters are generally greater than 100 μm.
- Better wall apposition (capture efficiency) by being able to adjust the filter diameter to suit the patient’s anatomy.
Dr. William Gray
References
- Carotid Artery Stenting in Octogenarians. Periprocedural Stroke Risk Predictor Analysis From the Multicenter Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events (CAPTURE 2) Clinical Trial Stroke. 2010; 41: 757-764 doi: 10.1161/STROKEAHA.109.569426; Stroke. 2007; 38: 707-714 doi: 10.1161/01.STR.0000250047.01624.fd
- Data on File at Contego Medical.
For product inquires in Europe: sales@contegomedical.com.
The Paladin System is CE marked and available in select European markets. Physicians are advised to consult the local product IFU prior to use.
