Paladin System Case Studies
Treatment of a Symptomatic Patient Using the Paladin System with FilterWire EZ™ Embolic Protection System (Boston Scientific) and the Roadsaver® Carotid Artery Stent (Terumo) at the Sankt Gertrauden Krankenhaus in Berlin, Germany by Ralf Langhoff, MD
A 71-year old male presented to Sankt Gertrauden Krankenhaus with a symptomatic right internal carotid artery in the right internal carotid artery (RICA) stenosis reporting symptoms of an ipsilateral TIA. The patient’s only significant medical history was hyperlipidemia treated with statin therapy. Preliminary neurological examination was normal, and the baseline NIHSS was 0/42. The patient underwent computed tomography angiography (CTA) which revealed an 80% lesion in the RICA of approximately 15 mm in length.
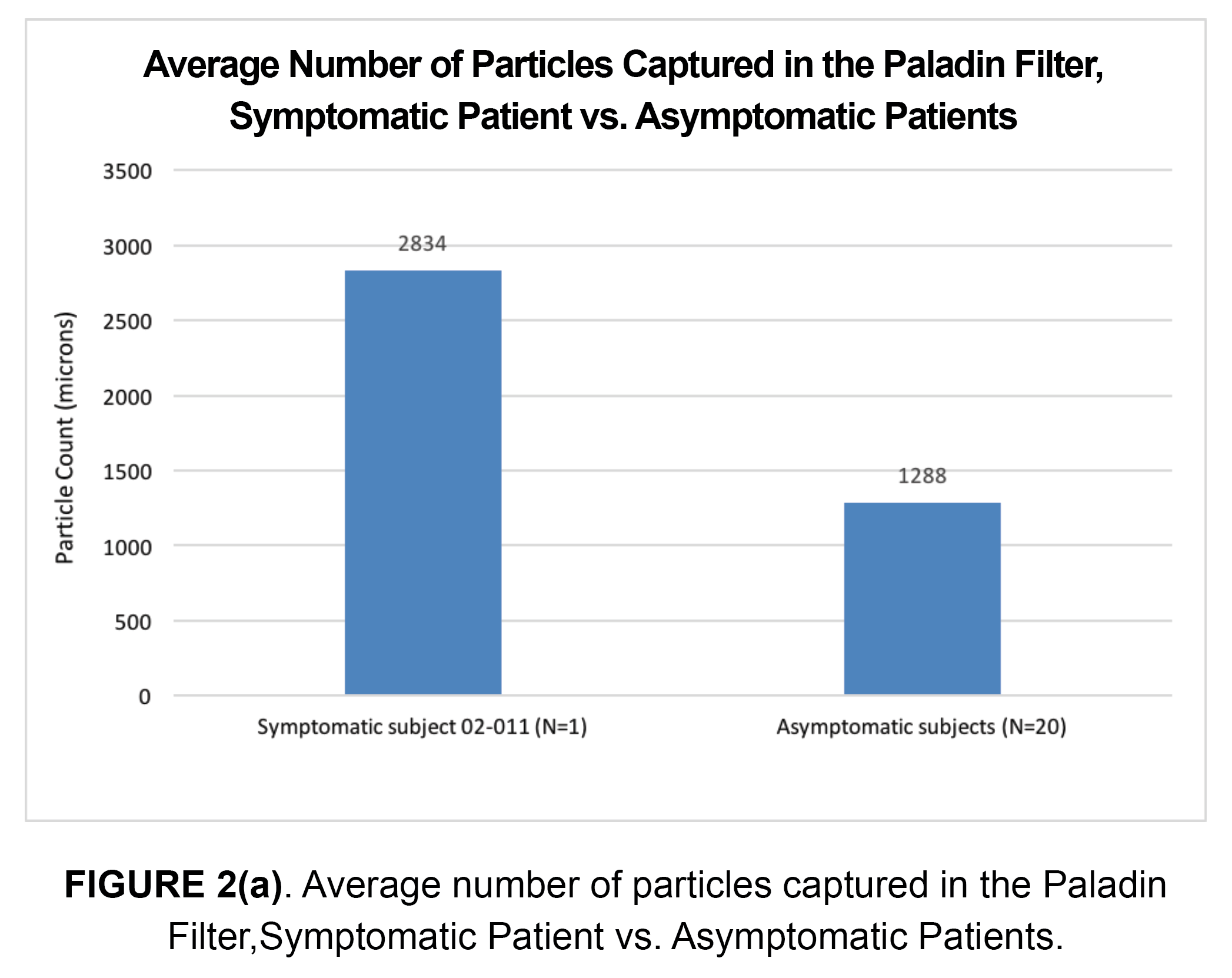
In this case of a patient with a symptomatic RICA stenosis, more embolic particles were captured in the Paladin filter (n=2,834) as compared to the mean number of embolic particles captured in 20 asymptomatic patients (n=1,288), Figure 2(a). These results are consistent with a known higher risk of embolization during post-dilation of symptomatic lesions as compared to asymptomatic lesions1.
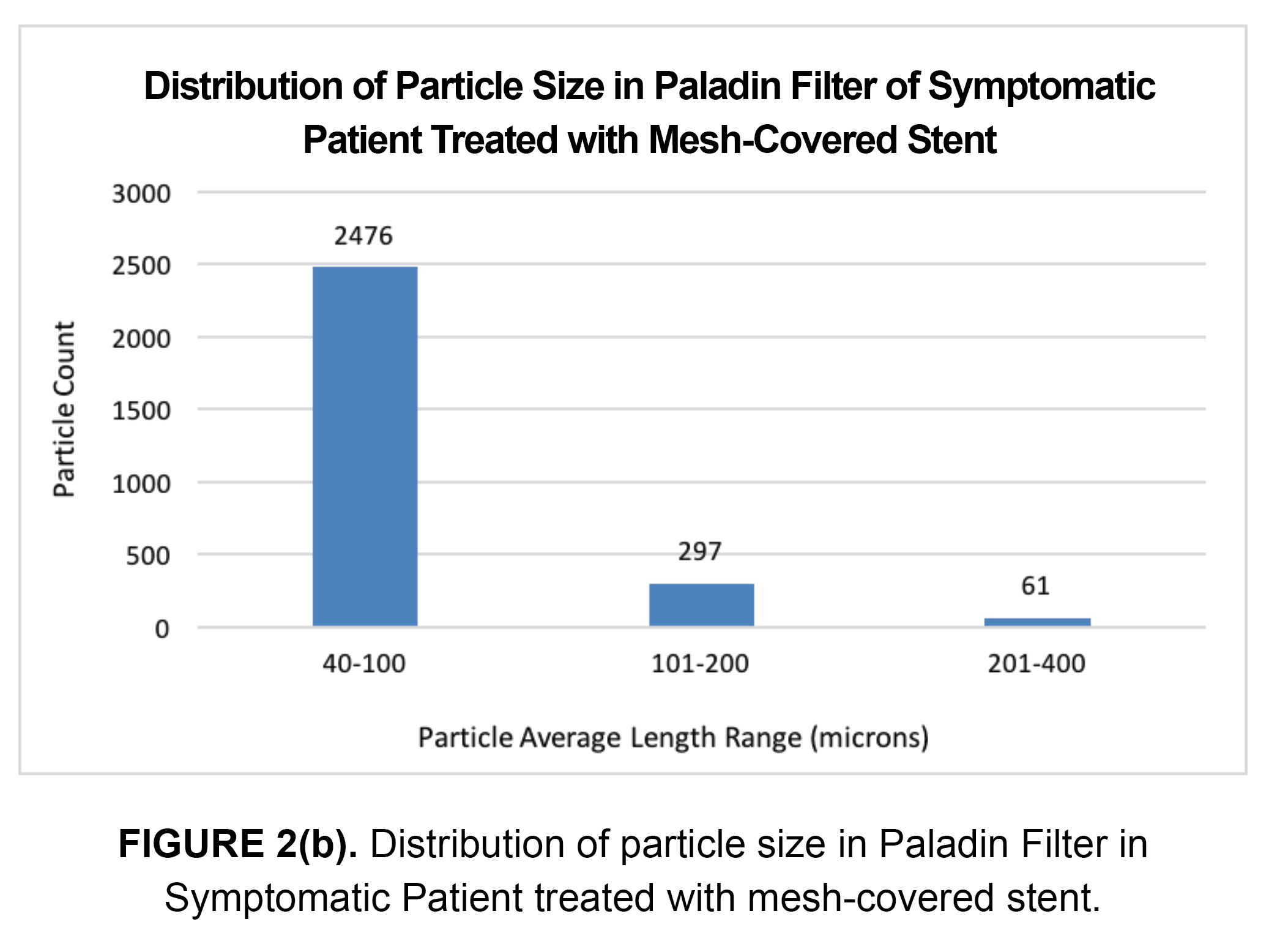
A further analysis of the 2,834 particles captured revealed that 2,476 (87%) particles were between 40 to 100 microns, 297 (11%) particles were between 101 to 200 microns, and 61 (2%) particles were between 201 and 400 microns. Results are shown in Figure 2(b). While this patient was treated with a mesh-covered stent, this did not appear to significantly reduce the number of embolic particles captured in the Paladin filter during post-dilation.
1Gray, W. A., et al. (2007), The CAPTURE registry: Predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Cath. Cardiovasc. Intervent., 70: 1025–1033. doi:10.1002/ccd.21359
Treatment of Previously Symptomatic Patient using Paladin System with Emboshield® NAV6 and XACT® Carotid Stent (Abbott Vascular) at Hamburg University Cardiovascular Center in Hamburg, Germany by Joachim Schofer, MD
A 61-year-old female presented to the Hamburg University Cardiovascular Center with a history of percutaneous transluminal angioplasty (PTA) and stenting of the left internal carotid artery (LICA) 5 years prior. The patient subsequently experienced motor aphasia which quickly resolved, however, diffusion-weighted magnetic resonance imaging (DW-MRI) revealed a new ischemic/embolic event.
Angiography revealed a target lesion stenosis of approximately 90% with lesion length of 15 mm and a reference vessel diameter of 5.0 mm. A primary distal protection device, Emboshield NAV6, was placed in the pre-petrous segment of the RICA. The RICA lesion was direct-stented with a 5 mm x 15 mm XACT carotid stent. A 5.0 mm x 20 mm Paladin System was then used to successfully post-dilate the stented lesion. Residual stenosis of the lesion was 20%.
The patient underwent DW-MRI scans prior to the procedure and within 24 hours post-procedure to assess for the presence of new ischemic lesions. The pre-procedure DW-MRI was unremarkable, with no cerebral ischemia. Post-procedure DW-MRI revealed no new lesions. The patient reported no major adverse events through the 30-day follow-up.
Paladin System Use with Mo.MA® Ultra Proximal Cerebral Protection Device (Medtronic) and the Roadsaver Carotid Artery Stent with Histological Evaluation of Filter Contents at Leipzig Heart Center in Leipzig, Germany by Dierk Scheinert, MD
An 80-year-old male presented to the Leipzig Heart Center with asymptomatic carotid artery stenosis of the RICA. The patient had a medical history of previous tobacco use, hyperlipidemia treated with statins, hypertension, valvular disease and renal insufficiency. The patient also had a previous percutaneous carotid intervention of the left carotid artery.
Following the procedure, the Paladin filter was removed from the catheter, placed into formalin and evaluated by a histology core laboratory to identify particulate debris collected (Figure 4). In total, 2,789 particles were collected in the Paladin filter, ranging in size from 40 μm to >200 μm. The largest particle detected in the Paladin filter was 374 microns in size.

Staged Bilateral Intervention using FilterWire EZ Embolic Protection System and the Roadsaver Carotid Artery Stent at Sankt Gertrauden Krankenhaus in Berlin, Germany by Ralf Langhoff, MD
An 81-year-old male presented to the Sankt Gertrauden Krankenhaus with asymptomatic bilateral carotid artery stenosis. The patient had a medical history of previous smoker, hyperlipidemia treated with statins, hypertension, renal insufficiency and a previous bilateral CEA.
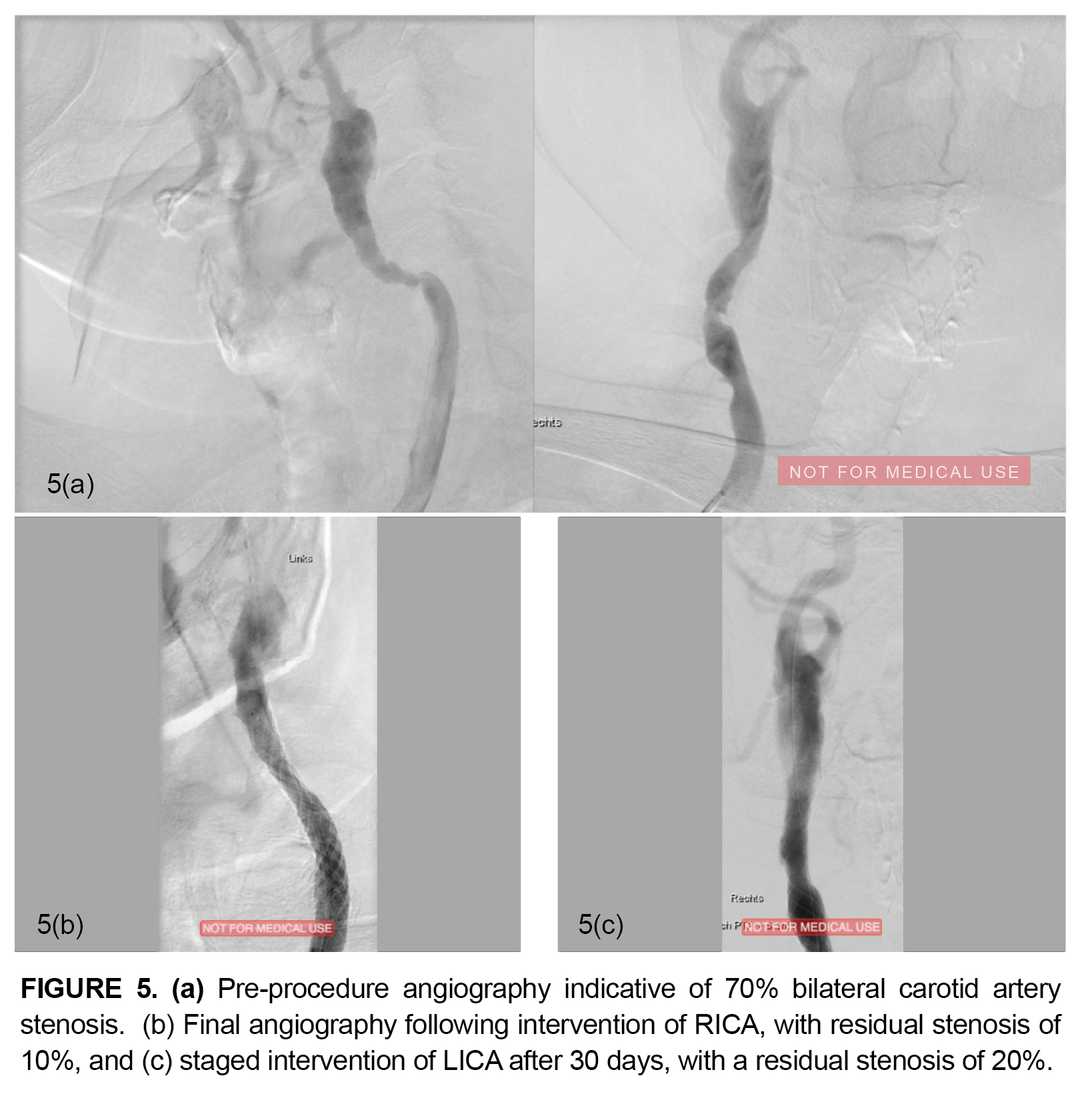
The patient returned for a staged intervention to the RICA following the 30-day follow-up period. A FilterWire EZ System was again chosen as the primary distal protection device. The lesion was treated with a 9 mm x 30 mm Roadsaver stent and post-dilation was successfully performed using a 5.0 mm x 20 mm Paladin System. Residual stenosis of the lesion was 20% (Figure 5c). No major or device-related adverse events were reported through the 30-day follow-up period.
Paladin System Use with SpiderFX™ Embolic Protection Device (Medtronic) and PRECISE® PRO Rx® Carotid Stent (Cordis) at The Cardiovascular Center in Bad Krozingen, Germany by Thomas Zeller, MD
A 67-year-old female presented to the Universitaets-Herzzentrum Freiburg – Bad Krozingen with an asymptomatic stenosis of the LICA.
The patient underwent the carotid intervention of the LICA. A SpiderFX Embolic Protection Device was used for primary protection. Pre-dilation of the lesion not was performed prior to stent placement and the LICA lesion was successfully treated with an 8 mm x 30 mm PRECISE stent. Post-dilation of the stent was performed using a 5.0 mm x 20 mm Paladin System. Residual stenosis of the lesion was 20%. No adverse events were reported during the procedure through 30-day follow-up.
Use with Emboshield NAV6 Embolic Protection System and XACT Carotid Stent with the Paladin System in Frankfurt, Germany by Horst Sievert, MD
A 67-year-old male presented to the Cardiovascular Center in Frankfurt with symptomatic LICA stenosis.
The patient had a medical history of hyperlipidemia treated with statins, hypertension, a previous posterior stroke and an abnormal ECG. Diagnostic angiography revealed a target lesion stenosis in the LICA of approximately 76% with a target lesion length of 15 mm and reference vessel diameter of 4.0 mm.
Read More
FILTER PARTICLE CONTENT ANALYSIS
A quantitative particle analysis was conducted on the Paladin System filter used for post-dilation of carotid artery stents in a subset of patients‡. All interventions were performed using a primary EPD. Paladin filters and primary EPD filters were removed from the catheter, placed into formalin and evaluated by a histology core laboratory (Imaging and Analysis, LLC, Saint Paul, MN).
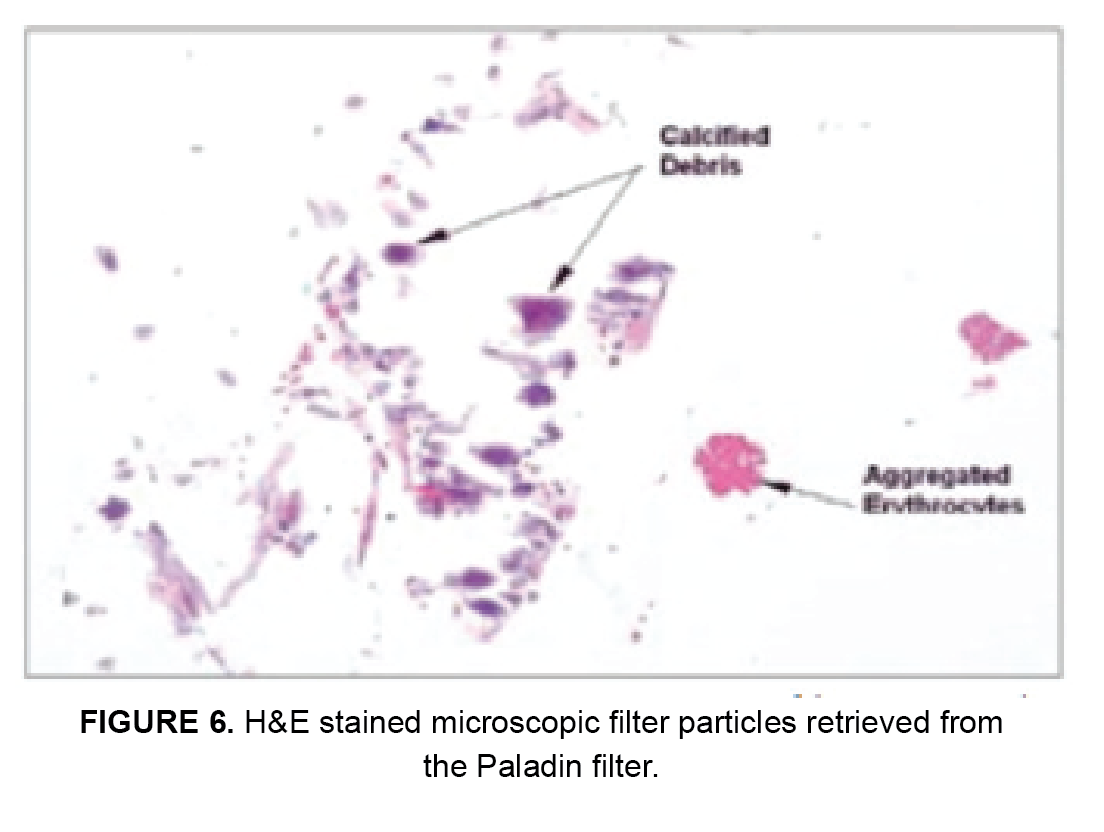
The preserved Paladin System and primary EPD underwent hematoxylin and eosin (H&E) stained microscopic evaluations to identify particulate debris collected (Figure 6). Filter particle count results are shown in Figure 7.
Microscopic debris was present in 100% of filters. The majority of particles (>85%) captured in the Paladin filter were 100 μm or less in size. The mean number of particles between 40 – 100 microns collected in the Paladin filter was 1,167 compared to a mean of 700 collected in the primary EPD, suggesting that the small-pore Paladin filter collects the smaller particles that could pass through the primary EPD, and may provide additional patient safety.
Available distal filter based embolic protection devices feature pore sizes of more than 100 microns, and thus are unlikely to capture a majority of embolic particles released during the post-dilation phase of carotid artery stenting. The small-pore filter on the Paladin System collects particles that could pass through the primary filter, thus providing additional patient safety during CAS during the time of highest risk of microembolization.
Ralf Langhoff, MD; Sankt Gertrauden Krankenhaus, Berlin, Germany.
Joachim Schofer, MD; Hamburg University Cardiovascular Center, Hamburg, Germany.
Dierk Scheinert, MD; Leipzig Heart Center, Leipzig, Germany.
Horst Sievert, MD; Cardiovascular Center, Frankfurt, Germany
Thomas Zeller, MD; Universitats-Herzzentrum Freiburg, Bad Krozingen, Germany.
Core lab analysis conducted by: Jerry Sedgewick, Imaging and Analysis, LLC, Saint Paul, MN.
Results from case studies are not necessarily predictive
of results in other cases. Results in other cases may
vary. See “Instructions for Use” for complete procedure
requirements. Patents pending.
XACT and Emboshield are registered trademarks of the Abbott Group of Companies.
Mo.MA® Ultra is a registered trademarks of Medtronic, Inc.
SpiderFX™ is a trademark of Covidien LP
Roadsaver® Carotid Artery Stent System is a registered trademark of Terumo Medical Corporation.
PRECISE® PRO Rx is a registered trademark of Cordis Corporation.
