
Medtronic is now the sole distributor of this product in the United States. Any claims or product information found on this page is now superseded by content available on medtronic.com/neuroguard.
Integrated Embolic Protection (IEP) platform. Captures emboli other protection mechanisms can’t.6
- Designed to safeguard against stroke & cognitive impairment associated with microembolization7-9
- Proprietary 40 micron filter pores are 3-4x smaller than traditional filters and mesh-covered stents6
- Physician-controlled filter is adjusted to the anatomy to help prevent particles from passing around the filter
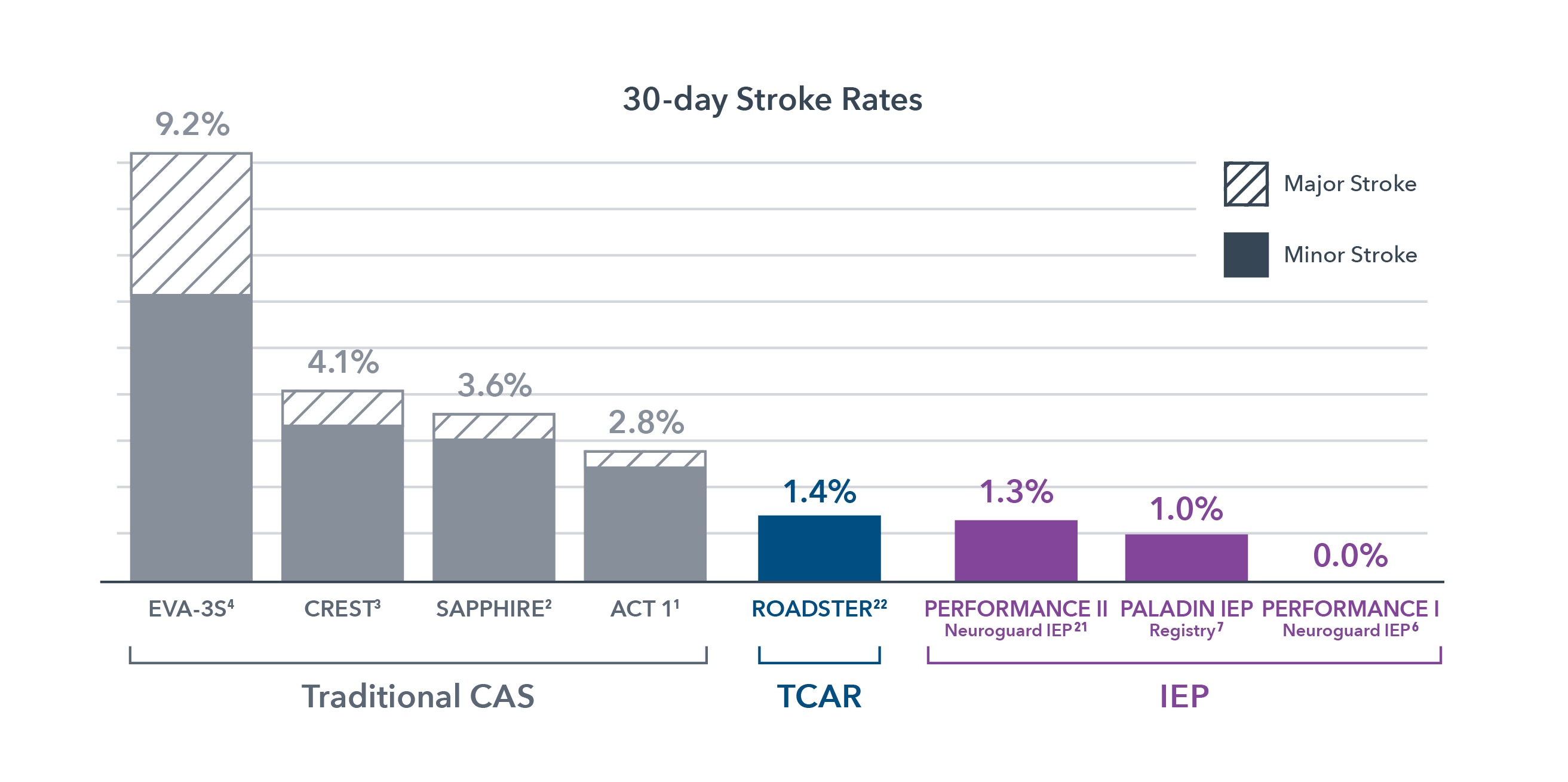
The difference between success and stroke is measured in microns
Designed for Precision: Built for precise and predictable stent deployment10 and vessel conformability
Built to Fit: Short landing zone means the system can be used on more than 94% of patient anatomies10
More Efficiency: 3-in-1 system reduces catheter exchanges and improves procedural efficiency10
Contego’s FlexRingTM technology provides the best of both open and closed cell stents with optimized flexibility AND radial strength.10
Let’s Get to ZeroTM
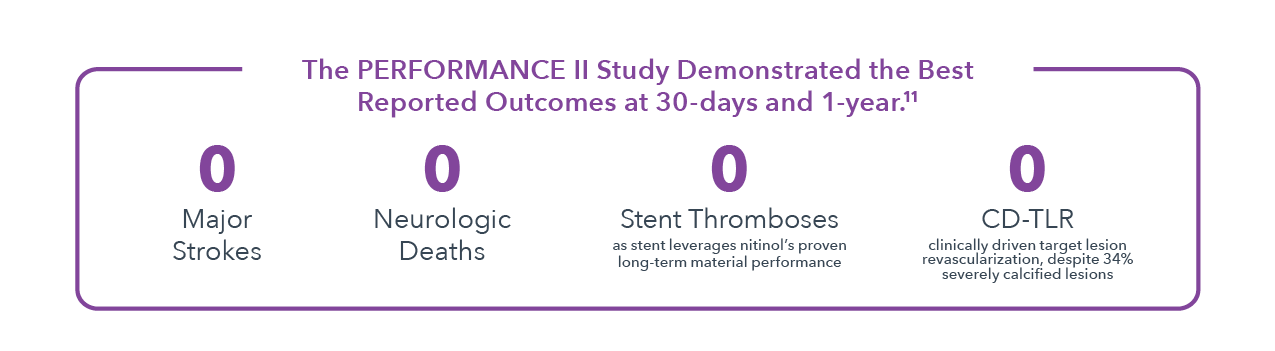
Clinical studies of IEP technology in CAS procedures have consistently shown unprecedented 99% freedom from strokes.1,2,11
The Neuroguard IEP System is approved for sale in the USA. Caution: Federal (United States) law restricts this device to sale by or on order of a physician. Prior to use, please see the Instructions for Use for a complete listing of Indications, Contraindications, Warnings, Precautions, Potential Adverse Events, Operator Instructions, and Directions for Use.
References
- Langhoff R, et al. Catheter Cardiovasc Interv. 2022;100(6):1090-1099.
- Langhoff R, et al. JACC Cardiovasc Interv. 2019;12:395-403.
- Petkoska D, et al. Cardiovasc Revasc Med. 2024;63:43-51.
- Henry M, et al. Catheter Cardiovasc Interv. 2007 Jun 1;69(7):1026-35.
- Ledwoch J, et al. Int J Cardiol. 2017 Jan 15;227:550-555.
- Per IFUs of devices: Pore sizes of various embolic protection devices: Angioguard 100µm, NAV6 120µm, and mesh-covered stents: CGuard 165µm, Terumo 375µm, Gore 500µm.
- Zhou W, et al. Jour of Vasc Surg. 2017 Mar;65(3):686-694.
- Hitchner E, et al. Jour of Vasc Surg. 2016 Dec;64(6):1719-1725.
- Maggio P, et al. Jour of Neuro Sciences. 2013 May 15;328(1-2):58-63.
- Data on File. Contego Medical, Inc.
- Gray W, et al. JACC Cardiovasc Interv. 2025;18(3)367-376.
- EVA-3S: Mas, Jean-Louis, et al. N Engl J Med 2006;355:1660-71.
- CREST: Brott et al, N Engl J Med 2010;363:11-23.
- SAPPHIRE Randomized: Yadav JS, et al. N Engl J Med 2004;351:1493-501.
- ACT 1: N Engl J Med 2016;374:1011-20.
- Kwolek CJ, et al. J Vasc Surg. 2015 Nov;62(5):1227-34.
